Work on tiny dots that light up TV screens and help doctors see the blood vessels that feed tumors has earned three scientists the 2023 Nobel Prize in chemistry.
Chemist Moungi Bawendi, chemist Louis Brus and physicist Alexei Ekimov split the prize for the discovery and synthesis of quantum dots, the Royal Swedish Academy of Sciences announced October 4.
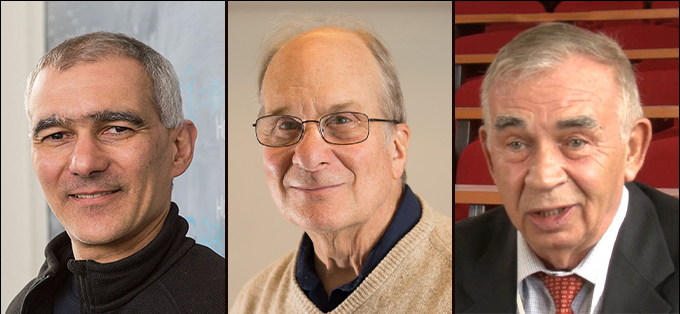
“Quantum dots are a new class of materials, different from molecules,” said Heiner Linke, a member of the Nobel committee. Just adjusting the size of these nanoparticles, roughly a few billionths of a meter across, can change their properties — optical, electric, magnetic, even melting points — thanks to quantum mechanics (SN: 6/29/15).
That’s also true of color. “If you want to make different colors with molecules, you would choose a new molecule, a new set of atoms” arranged in a different structure, Linke said. But quantum dots of different colors have the exact same arrangement of atoms. The only difference is particle size.
When quantum dots are irradiated by light, electrons within get energized, eventually releasing that energy as fluorescent light. The smaller the dots are, the more they compress the wave function of an electron, increasing its energy so that the dot appears blue. Larger dots appear red.
Dots of the same size made from different materials may also emit slightly different wavelengths of light, says Jean-Marc Pecourt, a chemist at CAS, a division of the American Chemical Society. Quantum dots are usually made from semiconductor materials, such as graphene, selenite or metal sulfides, Pecourt says. So by adjusting materials or the size of quantum dots, chemists can alter their properties for a wide variety of uses.
The idea that the size of these nanoparticles could alter their properties was predicted nearly a century ago, but at the time it seemed impossible to reproduce that effect in the real world. To do that, researchers would need a perfectly crystalline material, and would need to control the size of the nanomaterial very precisely, sculpting it atom layer by atom layer.
Then, in the early 1980s, Ekimov and Brus independently showed that it could be done. Ekimov, now at Nanocrystals Technology, Inc., in Briarcliff Manor, N.Y., demonstrated this in glass, adding copper chloride to produce tiny crystals and revealing that the color of the glass was linked to the size of those crystals. Brus, of Columbia University, made a similar discovery, but in a different context: He demonstrated the link between size and color for nanoparticles floating freely in a solution and in gaseous compounds (SN: 10/3/92).
Those discoveries triggered intense interest in how to harness these little dots for a variety of applications. But manufacturing them would require being able to control the size of the particles to precise specifications.
A decade later, Bawendi, of MIT, developed a method to precisely control the speed of the crystals’ growth in a solution, figuring out how to stop them right when they reach a desired size. He did this by first injecting chemical reagents into the solution that instantaneously formed the tiny crystals and then promptly adjusting the temperature of the solution, halting their growth.
“I’m deeply honored and surprised and shocked by the announcement this morning,” Bawendi said October 4 during an MIT news conference. “I’m especially honored to share this with Lou Brus, who was my postdoctoral mentor [from] whom I learned so much. I tried to emulate his scholarship and his mentoring style as a professor myself when I came to MIT.”
Bawendi started working on quantum dots after he met Brus at Nokia Bell Labs, headquartered in Murray Hill, N.J. The researchers needed high quality quantum dots to study the physics of the nanoparticles, Bawendi said. “It wasn’t because I wanted to make the best quantum dots possible for application, it was because we needed to make the best possible quantum dots to study them.” It took years of trial and error to work out the method, he said.
By making it possible to manufacture quantum dots, Bawendi’s method opened up a world of possible uses for the nanoparticles. Quantum dots make it possible to very precisely change the color of LED lights and dramatically improve their efficiency. Dots that glow with fluorescent light, injected into the body and attached to immune cells that swarm to cancerous tissues, can help surgeons distinguish even hard-to-see tumors (SN: 8/3/04). The ability to be tuned to absorb different wavelengths of light could also allow the manufacture of customized solar cells that are highly efficient in different light conditions. The dots might also be used to build quantum computers, Pecourt says (SN: 2/14/18).
Biomedical engineer and chemist Warren Chan says the prize is well deserved. “They’re the ones who built the foundation,” says Chan, of the University of Toronto. “I’m really happy that the field is getting credit for really changing the world, not just in quantum dots, but in a lot of different areas.”
One of the first applications came in the late 1990s when Chan and colleagues used quantum dots to tag cells in the lab, he says. “The surface modifications that were used for integrating quantum dots for applications were then also adapted for other types of nanoparticles.”
The Nobel committee looks not only at past contributions, but also the effect a discovery may have on the future, Chan says. The ability to tune nanoparticles by changing their size or surface properties could open a wide variety of possibilities that have not yet been explored. Chan and colleagues are now using quantum dots to detect infectious diseases, including HIV, influenza and hepatitis B.
“I was absolutely thrilled to see this,” says Judith Giordan, president of the American Chemical Society. “We have three people recognized who brought this technology from a dream, a hope, a theoretical construct … all the way through synthesis and manufacture.”
Earlier this week, the development of mRNA vaccines — widely speculated as a candidate for the 2023 chemistry Nobel Prize — received the Nobel in medicine or physiology instead (SN: 10/2/23).
“Sometimes chemistry gets a bad rap,” Giordan says. “But here are two magnificent examples of how chemistry has solved problems in the world.”
The three winners will share the prize of 11 million Swedish kronor, or about $1 million.











+ There are no comments
Add yours