Study uncovers stability hurdles in a promising lead-free solar cell material, signaling potential for a cleaner energy future.
Researchers have analyzed the properties of Cs2AgBiBr6, a material composed of cesium, silver, bismuth, and bromine, which holds promise for solar cell applications. With previous studies yielding conflicting data on its response to moisture, humidity, and sunlight, there arose a need for more detailed investigation to discern the material’s viability for efficient use in solar energy.
“During literature research, we found that there are very different reports on the stability of Cs2AgBiBr6,” explained Dennis Michael Jöckel, a materials scientist at the Fraunhofer Research Institution for Materials Recycling and Resource Strategies in Germany and lead author of the study, in an email. “Therefore, we decided to investigate the material’s behavior under different environments to close this research gap.”
Unlocking perovskite potential
This substance belongs to a group of naturally occurring minerals called perovskites, which possess a unique crystal structure. “Due to their compositional flexibility and structural tolerance and the excellent magnetic, electric and optical properties, perovskites are used for various applications such as sensors, LEDs, catalysts,” explained Jöckel.
In their study published in Advanced Photonics Research, Jöckel and his colleagues, which included Songhak Yoon, professor at Fraunhofer Research Institution for Materials Recycling and Resource Strategies, focused on the use of Cs2AgBiBr6 perovskite in solar energy — a crucial technology in the renewable energy sector.
“For the application in solar cells, perovskites work just like any other solar absorber in photovoltaics,” said Jöckel. “Their function is based on the photovoltaic effect. Here, an incoming photon excites a valence electron (outer electron) from the solid absorber material. If the photon’s energy is sufficient, the electron jumps to the conduction band (higher energy level). From the conduction band, the electron is led to the current collector, where it is then fed via the circuit to electrical consumers in the grid.”
An appealing aspect of Cs2AgBiBr6 perovskite for solar cells is its moderate environmental impact coupled with fairly high efficiency in converting solar radiation into electrical energy.
“Currently, the most efficient perovskite solar cells are based on combinations of lead iodides and bromides,” Jöckel explained. “Lead plays an important role here due to its optical and electronic properties. Unfortunately, most lead-based perovskites exhibit serious stability issues. Furthermore, lead is known to be extremely harmful for ecosystems and humans. These serious downsides led to the novel research direction of lead-free perovskites for solar cells.”
Working towards sustainable solar solutions
Due to its promise in solar energy, Cs2AgBiBr6 perovskite has been the subject of many scientific studies. Surprisingly, many have led to conflicting results regarding its stability under various environmental factors normally encountered during solar cell operation. These include exposure to air, moisture that can occur from rain, sunlight to which the cell must be constantly exposed, and overall aging.
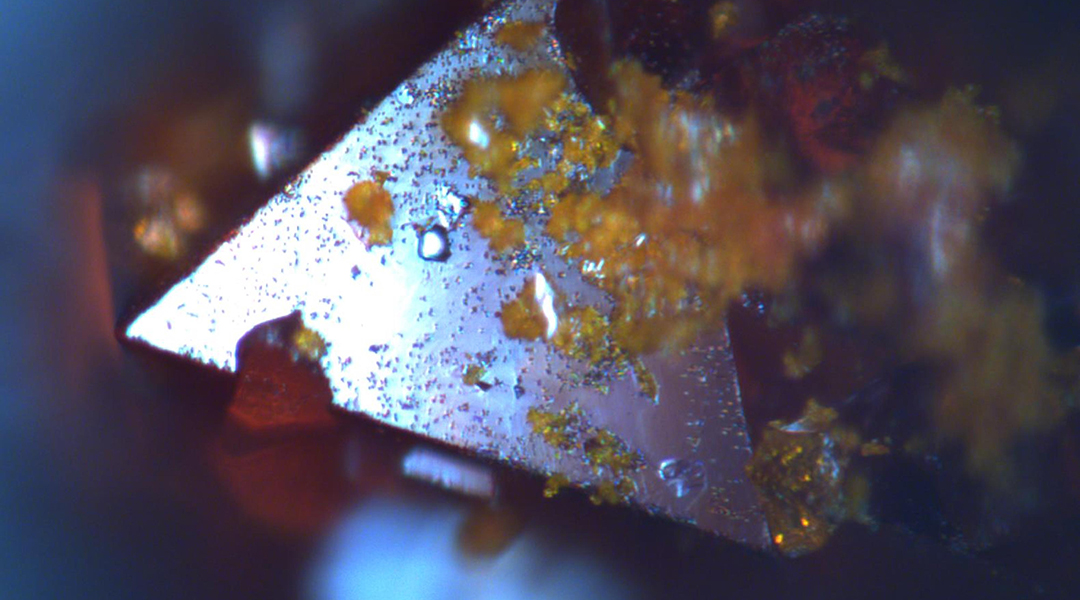
The team explored the influence of these factors on Cs2AgBiBr6 using a range of advanced spectroscopic techniques, including optical microscopy, ultraviolet and x-ray analysis, and found that under intense solar radiation, the material degraded, with many surface and bulk inhomogeneities forming on its surface as well as within the bulk material .When exposed to moisture, its degradation became even more significant and chemical decomposition of Cs2AgBiBr6 occurred, which led to a decrease in efficiency of the solar cells.
“Our most significant finding was proving the solar accelerated instability of the investigated material when exposed to different ambient conditions,” explained Jöckel. “We successfully demonstrated previously suggested degradation pathways.”
“Furthermore, to investigate the material’s potential for resource efficiency and scaling, we conducted the first regenerative synthesis of Cs2AgBiBr6 crystals from reused solvent to investigate potentials for sustainably scaling the material’s production,” he continued. “We concluded that for successful application in solar cells, the material’s [transparent casing] must be studied well to avoid solar accelerated degradation during the use phase of Cs2AgBiBr6-based solar cells.”
Despite proving these degradation issues, the researchers remain optimistic that Cs2AgBiBr6-based solar cells could one day be viable and that these challenges can be overcome.
“The next steps for Cs2AgBiBr6 are to mitigate our reported stability issues, which we believe is possible via sealing the structure from moisture and humidity,” concluded Jöckel. “Furthermore, the material’s power conversion efficiency in solar cell applications needs to be addressed in order for the perovskite to keep up with lead-based alternatives.
“In addition to the improvement of properties, the ecological footprint of these technologies needs to be assessed to ascertain that technologies like lead-free perovskite solar cells can really provide an environmental benefit.”
Reference: Dennis Michael Jöckel et al, Solar Degradation and Stability of Lead-Free Light Absorber Cs2AgBiBr6 in Ambient Conditions, Advanced Photonics Research (2024). DOI: 10.1002/adpr.202300269







+ There are no comments
Add yours